In this edition of the automobile technology segment, we bring to you the relevance, classification and functioning of catalytic converters. Before we begin to explain what catalytic converters are, let us discuss what the exhaust gas emissions from a vehicle consist of. A regular fuel (petrol or diesel) is made up of hydrocarbons. That means atoms of Hydrogen and Carbon arranged in various proportions form molecules of petrol or diesel. Essentially, after the treatment of natural resources, the end product we get is the hydrocarbon chains. Depending on the number of carbon atoms and length of the chain, it is either classified as petrol or diesel.
Also read: How do Variable Valve Timings and Lift improve efficiency of an engine?
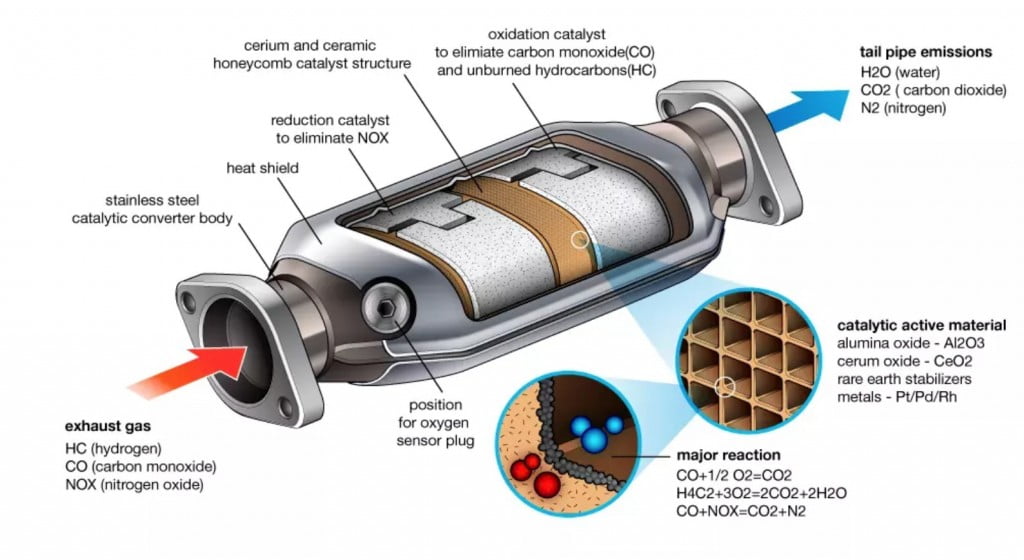
Why are Catalytic Converters needed?
During the combustion phase inside the cylinder of the engine, the air-fuel mixture almost never burns completely. Complete combustion is referred to as when the end products of the combustion are Carbon Dioxide and Water. But during the operation of an internal combustion engine, due to various reasons and factors, complete combustion almost never occurs. What that translates to is there are numerous undesirable and toxic gases present in the exhaust system of an engine. These gases are not only bad for environmental pollution but also cause severe health hazards if people inhale these. Being toxic and poisonous in nature, these gases are allowed in minute amounts according to the BS6 emission norms, which came into effect in 2019.
Also read: What are exhaust gas components? What is exhaust scavenging and its advantages?
The trend around the world is the same as well. Almost every year, the emission regulations are becoming stricter than the year before, as more and more people and governments are becoming environmentally conscious. The aim is to get rid of the internal combustion engines completely and switch to electric vehicles entirely. But obviously, that aim is too far away from the realities that we live in currently. Also, it is not possible to switch to EVs in a matter of few years. It will take a long time, but in the meanwhile, we have to keep the targets challenging so that the ultimate aim is achieved sooner rather than later. This requires a ton of technology to keep emissions in check and comply with so minute allowance for harmful gases like Carbon Monoxide, Oxides of Nitrogen and unburnt fuel (hydrocarbons).
Also read: How does combustion work in an engine? What are the uses of Spark Plugs and Fuel injectors?
Purpose of Catalytic Converters
This is where the Catalytic Converters come into the picture. The Catalytic Converters react with the harmful exhaust gases and the resultants formed are harmless gases, that are normally found in the atmosphere. This includes conversion of carbon monoxide to carbon dioxide and oxides of nitrogen to nitrogen and oxygen gases. The unburnt fuel (hydrocarbons) are also reduced to carbon dioxide and water and removed from the engine into the atmosphere. All the gases are ultimately the same gases that are found otherwise in the atmosphere as well. This is the sole purpose of the catalytic converters in the vehicles. Depending on the complexity, there are various forms of catalytic converters.
Also read: What are engine remapping, tuning and calibration? Should you remap your car?
Types of chemical reactions in the exhaust system
Principally, there are two kinds of chemical reactions that take place inside the exhaust system of an internal combustion engine. These are reduction and oxidation. How exactly do they occur, let us find out.
Reduction Reactions
There are metals that act as catalysts to make the chemical reaction occur instantly. These are Platinum and Rhodium. When the exhaust gases flow over the surface of the catalytic converter, the oxides of nitrogen get reduced to individual Nitrogen and Oxygen atoms. This is a simple chemical reaction that you must’ve studied during your school days. The end products, Oxygen and Nitrogen are harmless and are emitted out to the environment.
Also read: Types of clutches – Dry, Wet, Single-plate and Multi-plate – Pros and Cons of each!
Oxidation Reactions
The catalysts used inside the catalytic converter to assist the chemical reaction are Platinum and Palladium and the simple reaction occurs. The atoms of Nitrogen and Oxygen coming out of the reduction reaction combine with Carbon Monoxide and unburnt Hydrocarbons to give carbon dioxide and water vapours as the end products. In this way, all the three major aspects of the exhaust emissions, namely, Carbon Monoxide, Oxides of Nitrogen and unburnt hydrocarbons are treated in the catalytic converter and the pollution to the environment is minimized.
Two-way Catalytic Converter
A two-way catalytic converter refers to that, which can only perform two tasks out of the three mentioned above. These are hydrocarbons and carbon monoxide. The NOx emissions are not treated in this catalytic converter. For that, there are other systems in place in addition to the two-way catalytic converter which treats the oxides of Nitrogen in the exhaust system. One of the examples of that is the Selective Catalytic Converter.
Also read: Types of turbochargers – VGT, Twin-Scroll, Twin-Turbo, Sequential Turbos and E-Turbos!
Selective Catalytic Converter (SCR)
A Selective Catalytic Converter requires an additional storage tank filled with Urea. After the two-way catalytic converter, there is an injector that is controlled by the ECU (Electric Control Unit) of the vehicle. The ECU signals the injectors to spray a particular amount of urea into the exhaust manifold depending on the data that the ECU receives from the sensors of the engines. Accordingly, the amount of urea required to convert oxides of nitrogen into individual Nitrogen and Oxygen atoms is injected. Because this system is a bit complicated and requires additional storage and refilling of urea in the vehicle, it is a bit expensive and therefore, used only in above the entry-level budget cars.
Also read: What is Gasoline Direct Injection ? Why is it relevant in modern cars?
Three-way Catalytic Converter
A three-way catalytic converter is one that is used mostly in vehicles. All three reduction and oxidation reactions occur inside the single catalytic converter and there is no need to bring in another chemical or component. The working efficiency of the three-way catalytic converter is generally around 90% which is sufficient in almost all environments.
Also read: All you need to know about 4WD and AWD – What is a Differential?
You must have heard the news recently that many carmakers in India have completely gotten rid of the diesel engines from their fleet. Internationally as well, a lot of OEMs have stopped the manufacturing of diesel engines completely. This is because a diesel engine would require the technology that we mentioned earlier, Selective Catalytic Reduction which makes the cars a lot expensive. On top of that, the adoption of turbocharged petrol engines has rendered the point of diesel engines almost useless. One can easily get almost the same level of low-end torque as in the diesel engines and petrol engines require much less complication and less expensive three-way catalytic converters to keep all the toxic and harmful exhaust gases in check. The future, however, is moving towards even cleaner forms of energy like electrification. There is some form of electrification in a ton of vehicles even today and completely electric vehicles are slowly making their way into the mainstream automobile market around the globe.
Image Credit: Cars.com
The post What Are Catalytic Converters? How Do They Reduce Emissions? appeared first on Car Blog India.
from Car Blog India https://bit.ly/38H9ZEx
Are you looking to get paid quick Cash for Catalytic Converter in Australia? Have you heard about Catalytic Converter Buyers? We are fully licensed and customer friendly cat Converter Buyers paying maximum Cash for Catalytic Converter in Australia with free pick up service. Call us if you want to get rid of it or sell it ASAP. We are best in metal recycling and hence buy all types of cat Converter for recycling.
ReplyDelete